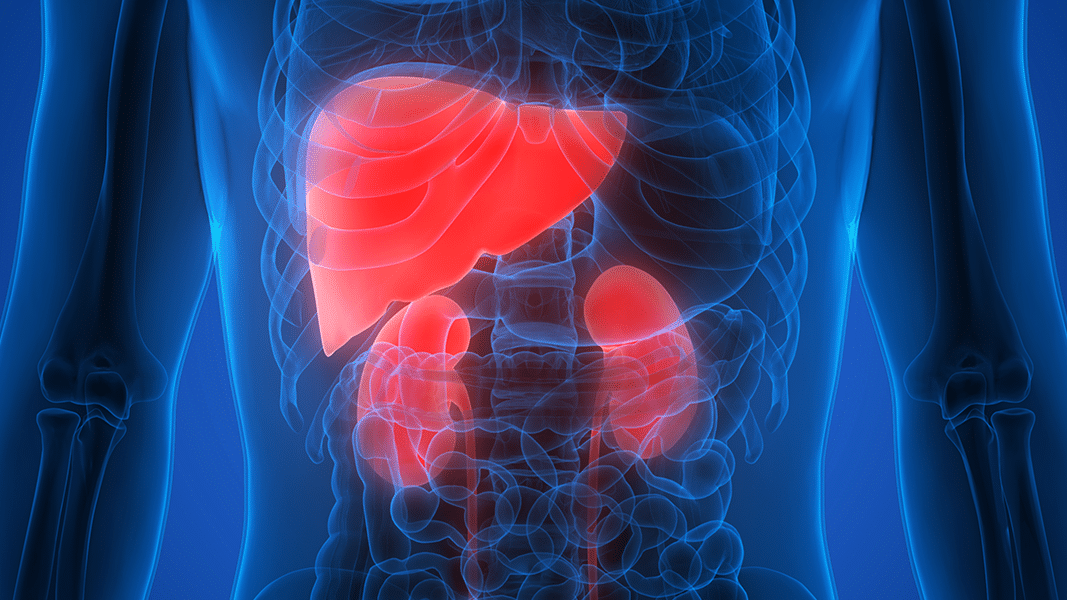
Epidiolex makers studied how liver impairment affected metabolism of CBD. They found that while hundreds of mgs of CBD were still safely tolerated, it was metabolized more poorly. Doses should be lowered depending on the severity of liver impairment.
Dosing cannabinoids is a conundrum. Anything between 5-500 mg CBD may considered a “reasonable” dose in different contexts. Finding an optimal dose is even more difficult for people with liver or kidney diseases, as these are the major organs that clear chemicals from the blood. So the makers of Epidiolex, an FDA-approved CBD pharmaceutical, studied how patients with liver impairment should be dosed. CBD can still be used safely, according to the article published in the Journal of Clinical Pharmacology, but the dose needs to be lower than in healthy individuals. The researchers asked thirty people to swallow 200 mg CBD then measured the cumulative exposure to CBD and its metabolites. Poor liver function cause a significant increase in CBD exposure. It was 2.5 times larger for those with moderate liver dysfunction, and over 5 times larger in those with severe impairment. The exposure to one of CBD’s active metabolites, 7-OH-CBD, was also inversely correlated with participant’s liver health. There were no serious adverse events in this small study, implying people with liver impairments can safely use CBD, though they should start with a lower dose and titrate more slowly. Liver capability did not influence the time it took for CBD to get through the gut: CBD’s concentration in the bloodstream peaked 2-3 hours after ingestion. (All participants ate two hours before ingesting CBD.) The researchers controled and tested for sex differences (none were apparent), but all participants were white. This is not uncommon, especially in small pilot studies such as these, but it’s a deep-rooted problem. The cumulative nature of research means that such choices build up and bias medicine.
Adrian Devitt-Lee is a research scientist and longtime Project CBD contributor. © Copyright, Project CBD. May not be reprinted without permission.