Ancient peoples attuned to ecological subtleties referred to certain consciousness-altering plants and fungi as “teachers.” What has cannabis taught humankind?
Long before the written word, cannabis figured prominently in the shamanistic traditions of many cultures, which found uses for virtually every part of the plant. The stalk provided fiber for cordage and cloth; the seeds, a rich source of protein and essential fatty acids, were eaten as food; and the roots and resinous flower tops were used in medicinal and ritual preparations.
What accounts for the herb’s broad and enduring appeal? Scientific efforts to pinpoint the psychoactive ingredients that cause the mild euphoria beloved by cannabis enthusiasts began in the 19th century. But investigators were stymied by the complex, lipophilic (oily) nature of the plant, which required sophisticated technology to probe and parse.
A key turning point for modern cannabis research came in 1964, when Israeli scientists Raphael Mechoulam and Yechiel Gaoni isolated and identified tetrahyrdocannabinol (THC) as the high causer. Mechoulam also elucidated the chemical structure of several other cannabis components, including cannabidiol (CBD), an intriguing, non-intoxicating molecule. He called these unique botanical compounds “cannabinoids” and likened the plant to “a pharmacological treasure trove.”
The buzz about THC, the queen bee of cannabinoid pharmacology, was the main impetus for scientists who sought to understand how marijuana conferred its psychoactive effects. What happens in the brain that makes people feel high? Or hungry? Or calm? Or slightly less encumbered by life’s difficulties? Animal studies focusing on THC provided a foundation for investigating its mechanism of action on a molecular level. Another quarter of a century would pass before cannabis, the plant teacher, led researchers to one of the great scientific discoveries of all time – actually, a series of discoveries – that revealed the existence and inner workings of a protective, body-wide regulatory system activated by cannabinoid compounds.
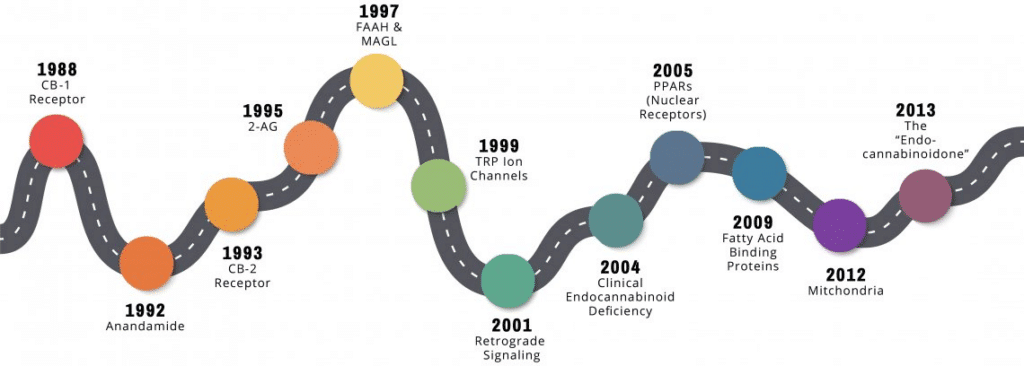
Endocannabinoid Discovery Timeline
Part 1: The Canonical Endocannabinoid System
1988: CB1 Receptor
The big breakthrough came in 1988, when scientists at the St. Louis University Medical School determined that a rat’s brain has receptor sites – specialized protein molecules embedded in cell membranes – that are activated by THC. Initially identified by Professor Allyn Howlett and her graduate student William Devane, and cloned two years later, this cannabinoid receptor, dubbed “CB1,” turned out to be far more abundant in the mammalian brain than any other G-protein-coupled receptor (GPCR).
Nearly half of all U.S.- approved pharmaceuticals target GPCRs, which comprise a super-family of over 800 different human receptors that share the same basic protein structure – hundreds of amino acids strung together in a crumpled chain, crisscrossing the cell membrane seven times. CB1 receptors are concentrated in the mammalian brain and central nervous system. Subsequent research showed that CB1 receptors are also present to a lesser extent in the gut, skin, and various internal organs. All animals with a spinal cord (and going back even earlier to the ancient sea squirt) have CB1 receptors. CB1 signaling would prove to be critical for regulating numerous physiological processes, including the body’s stress response and how we experience pain.
The discovery of the CB1 receptor would have huge implications for nearly every area of medical science. It opened the floodgates of research into our innate cannabinoid biology. Why do we have receptors that are capable of responding to plant cannabinoids such as THC? Scientists realized there had to be an endogenous, THC-like compound, our inner cannabis, so to speak, that signaled through these receptors. The search was on for CB1’s internal trigger.
1992: Anandamide
Enter N-arachidonoylethanolamine, the first endogenous cannabinoid neurotransmitter identified by scientists. (A neurotransmitter is a chemical that nerve cells use to send signals to other neurons.) In 1992, a trio of researchers at Hebrew University in Jerusalem – Raphael Mechoulam, William Devane, and Lumir Hanus – isolated a novel lipid neurotransmitter that binds with the CB1 receptor in pig brain tissue. They called it “anandamide,” Sanskrit for bliss, a word suggestive of its mood-altering effects.
Although anandamide and THC don’t share a similar molecular structure, they behave in a similar way when they bind to the CB1 receptor, somewhat like a key fitting into a lock. Anandamide, the endocannabinoid, and THC, the phytocannabinoid, are both signaling molecules (ligands) that turn on CB1, initiating a cascade of changes within cells that regulate an astonishing range of physiological functions, including appetite, mood swings, glucose metabolism, pain perception, even fertility. High levels of anandamide are crucial for ovulation, and fluctuations of anandamide levels during the gestational cycle can affect fetal development.
Cells create anandamide “on-demand,” whenever our bodies need to stay even keel during stressful interludes. Subsequent studies would show that physical exercise boosts anandamide levels, resulting in the “runner’s high.” By binding to CB1, anandamide protects neurons and facilitates neurogenesis, the creation of new brain cells in adult mammals. Every animal with a nervous system produces anandamide.
1993: CB-2 Receptor
Scientists identified a second type of cannabinoid receptor – “CB2” – which is present throughout the immune system, the peripheral nervous system, metabolic tissue, and in many internal organs. Initially reported in Nature in 1993, this discovery shed new light on how cannabinoid signaling regulates inflammation and why cannabinoid therapy could be a helpful treatment for a raft of autoimmune diseases. Aberrant CB2 receptor signaling is implicated in metabolic syndrome, peripheral neuropathy, insulin resistance, liver disease, and other inflammatory conditions.
CB2 receptors are found in all immune cells, including microglia and astrocytes, which modulate immune function in the brain. For the most part, however, CB2 receptors are expressed much less than CB1 in the central nervous system. But CB2 is significantly upregulated (kicks into high gear) in response to a brain injury or a neurodegenerative condition, such as Alzheimer’s or multiple sclerosis.
THC stimulates both types of cannabinoid receptors. However, when THC binds to CB2, it does not trigger the psychoactive high that cannabis is known for because CB2 receptors are not concentrated in the brain. THC binding to CB1, the abundant central nervous system receptor, causes the high. Consequently, researchers set their sights on healing without the high by developing drugs that stimulate the CB2 receptor, while bypassing CB1. But anandamide, the endocannabinoid that binds to CB1, actually has very little binding affinity for CB2 – which means there must be another natural compound, an endogenous ligand, produced by the body that activates CB2 receptors.
1995: 2-AG
Found in canine gut tissue, 2-Arachidonoylglycerol – or 2-AG for short – was identified as an endocannabinoid by Dr. Mechoulam and his team, and also by Japanese scientists, in 1995. Compared to anandamide, 2-AG proved to be more potent, more prevalent, and more broadly expressed throughout the body. 2-AG levels in the human brain are approximately 170 times higher than those of anandamide, and 2-AG binds efficiently to both cannabinoid receptors, CB1 and CB2.
Anandamide and 2-AG are both lipid neurotransmitters that signal all over the brain and body to help maintain internal homeostasis amidst a barrage of ever-changing environmental inputs. As the principal endogenous ligand for both CB1 and CB2, 2-AG plays a major role in regulating immune function. It reduces the expression of pro-inflammatory cytokines and reins in overactive immune cells. 2-AG levels in the brain surge after a head injury or a stroke.
Like anandamide, 2-AG is involved in modulating a wide range of mental and physiological processes. While they are similar and complementary in many respects, there are specific functional differences between the two endocannabinoids. Anandamide and 2-AG both protect cells against oxidative damage, and both compounds are adaptive in response to stress – but in distinct ways. And they are created and deactivated by different metabolic enzymes.
1997: Metabolic Enzymes – FAAH and MAGL
Endocannabinoids are born and broken down by various biosynthetic and catabolic enzymes. Thanks to these metabolic enzymes, endocannabinoids are made when needed and then degraded after serving their purpose. Anandamide is broken down by FAAH [fatty acid amide hydrolase], while 2-AG is deactivated primarily by MAGL [monoacylglycerol lipase]. The molecular structure of FAAH was characterized by Ben Cravatt at the Scripps Research Institute in 1996, and the following year Italian scientists identified MAGL as a key degradative enzyme for 2-AG.
Metabolic enzymes regulate endocannabinoid activity by controlling anandamide and 2-AG levels. Because anandamide & 2-AG degrade rather quickly, blocking their enzymatic metabolism – by inhibiting FAAH or MAGL – can elevate endocannabinoid levels and extend cannabinoid receptor signaling, with consequent neuroprotective benefits. Variations in the genes that code for FAAH and MAGL are associated with divergent health outcomes; too much of either enzyme can deplete endocannabinoid tone, resulting in what some would call a “weak constitution.”
The cloning of FAAH and MAGL marked a decade since the momentous discovery of the CB1 receptor, which really got the ball rolling in terms of cannabinoid science. The two cannabinoid receptor subtypes along with anandamide, 2-AG, and their biosynthetic and degradative enzymes, comprised the basic components of the canonical or “classical” endocannabinoid system, which modulates most biological functions. The endocannabinoid system plays a pivotal role in maintaining a healthy, stable environment inside the body, despite fluctuating external inputs and stressors. In the years ahead, new research would deepen our understanding of this ubiquitous lipid signaling ensemble.
Part 2: Foraging the Neuronal Forest
1998: Entourage Effect
The phrase “entourage effect” first appeared in a July 1998 science paper by S. Ben-Shabat and several colleagues. Published in the European Journal of Pharmacology, the article focused on 2-AG and “a novel route for molecular regulation of endogenous cannabinoid activity.” The authors reported that 2-AG’s binding affinity for CB1 and CB2 was enhanced by the presence of other endogenous lipid compounds that were not, strictly speaking, part of the canonical cannabinoid framework. A subsequent paper that year in the same journal discussed “the effects of ‘entourage’ compounds on the activities of anandamide and 2-arachidonoyl glycerol.”
A scientific phrase intended as a reference to the holistic, interactive underpinnings of the endocannabinoid system was subsequently applied to the complex chemical make-up of herbal cannabis. Just as endocannabinoids don’t act in isolation, neither do plant cannabinoids. The effects of THC and CBD are influenced by dozens of aromatic terpenes, flavonoids, and minor cannabinoids that may be present in a given cultivar. Each of these compounds has specific medicinal attributes, but when combined they create an entourage or ensemble effect so that the therapeutic impact of the whole plant (flower or essential oil) is greater than the sum of its isolated components.
The notion of an entourage effect implicitly called into question the primacy of monomolecular medicine favored by pharmaceutical firms and government regulators. It also pointed beyond the canonical endocannabinoid system to a wider schema that encompassed more than a pair of receptors, their ligands, and related enzymes. By highlighting the interplay between endocannabinoids and other lipid signaling molecules, pioneers in the burgeoning field of cannabinoid science pushed the conceptual envelope and opened the door to new vistas of understanding human biology and physiology.
1999: TRP (“Trip”) Ion Channels
Scientists developed research tools to probe and modulate various aspects of the endocannabinoid system. By administering synthetic “antagonist” compounds to block the CB1 receptor, scientists discerned that some of anandamide’s effects did not involve this receptor. In 1999, a team of European researchers reported in Nature that the ability of anandamide, a vasodilator, to relax blood vessels was mediated by its interaction with the “TRPV1” vanilloid receptor. Subsequent studies determined that 2-AG is also active at the TRPV1 receptor, which is instrumental in regulating core body temperature and inflammatory pain.
CBD also binds directly to TRPV1 – but not like a key fitting into a lock. TRPV1 is one member of a large, ancient family of Transient Receptor Potential ion channels, otherwise known as TRP (“trip”) receptors, which function as cellular sensors in response to heat, light, sound, pain, physical pressure, and other basic visceral sensations. Several TRP channels are modulated by endocannabinoids and plant cannabinoids, including CBD, CBDA, THC, THCA, THCV, CBG, CBC, and CBN.
The properties of many medicinal herbs are also mediated by TRP receptors. Capsaicin (hot pepper) binds to TRPV1. Mustard oil and other pungent spices activate TRPA1. And TRPM8 communicates the cooling sensation of menthol and peppermint. The revelation that anandamide blocks TRPM8 and stimulates TRPV1 was clear evidence that endogenous cannabinoids have a wider range of molecular targets than just the CB1 and CB2 receptors.
2001: Retrograde Signaling
Three groups of scientists published papers in 2001 showing that endocannabinoids engage in a unique form of intracellular communication known as “retrograde signaling.” Whereas other neurotransmitters typically travel in one direction from the signaling cell across the synapse (gap) to the receiving cell, anandamide and 2-AG both transit in the other direction – from the post-synaptic receiving cell to the pre-synaptic sender. That’s why endocannabinoids are referred to as “retrograde messengers.” They play a key role in managing how fast (or slow) other neurotransmitters fire.
Too much excitation can damage or destroy a cell. In response to a surge of glutamate, the brain’s main excitatory neurotransmitter, the post-synaptic receiving cell releases endocannabinoids, which travel backwards across the synaptic cleft to bind with a cannabinoid receptor on the sending cell that’s generating the glutamate. The CB1 receptor tells the presynaptic cell to turn down the glutamate volume. Retrograde signaling in the prefrontal cortex, amygdala, and hypothalamus alleviates overstimulation of the “HPA axis,” which governs the stress response. Endocannabinoids can also “disinhibit” (promote excitation) by suppressing synaptic activity involving GABA, a major inhibitory neurotransmitter. In essence, the retrograde mechanism functions as a dynamic, bidirectional feedback loop that fine-tunes synaptic transmission by putting the brakes on excessive physiological activity.
Just as the immune system protects against viruses and other pathogens, the endocannabinoids, as retrograde messengers, protect the brain against overstimulation, inflammation, and trauma. Prior to 2001, retrograde signaling was understood to occur only during the embryonic development of the brain and central nervous system. Researchers have since learned that endocannabinoids regulate embryonic and adult neurogenesis (the creation of new neurons in the brain), as well as stem cell migration.
2004: Clinical Endocannabinoid Deficiency
Dr. Ethan Russo, a neurologist and cannabinoid scientist, introduced the concept of “clinical endocannabinoid deficiency” in 2004. He hypothesized that diminished endocannabinoid function is at the root of several pathologies. Russo specifically mentioned four diseases – migraines, irritable bowel, fibromyalgia, and clinical depression – which often appear as a comorbid cluster of symptoms in patients who are cannabinoid deficient. Subsequent studies would lend credence to Russo’s thesis by linking endocannabinoid deficits to various aberrant conditions, including epilepsy, PTSD, autism, alcoholism, and other neurodegenerative ailments.
Several factors contribute to endocannabinoid dysfunction. Some are genetic: scientists have identified polymorphisms or mutations in amino acid sequences that encode the cannabinoid receptors and the metabolic enzymes that regulate endocannabinoid levels. Compelling evidence suggests that specific genetic variants may in some cases dictate health outcomes or, more likely, predispose one to certain diseases.
Epigenetic inputs – poor diet, lack of exercise, lousy sleep, drug abuse, racism, poverty – are also paramount, and in some ways more consequential in terms of fostering chronic stress. A significant risk factor for many maladies, chronic stress depletes endocannabinoid tone, leading to inflammation, hypertension, elevated cortisol levels, hormone imbalances, heightened blood sugar, cognitive impairment, and increased susceptibility to illness. It makes sense that cannabis therapeutics and other holistic healing modalities that enhance cannabinoid receptor signaling may be viable treatment strategies for clinical endocannabinoid deficiency disorders.
Part 3: Way Beyond Within
2005: PPARs – Nuclear Receptors
Researchers continued to discover therapeutic actions of endocannabinoids and plant cannabinoids that are not mediated by either CB1 or CB2. A 2005 article in Life Science, for example, reported for the first time that cannabinoid compounds bind to “PPAR-gamma,” a receptor situated on the surface of the cell’s nucleus.
PPAR-gamma is part of a family of lipid-sensitive peroxisome-proliferator-activated receptors, which regulate gene expression, lipid metabolism, and energy storage. Anandamide and 2-AG both activate PPAR-gamma; so does CBD. Preclinical probes indicate that PPAR-gamma activation reduces amyloid-beta plaque in the brain (related to dementia); prevents insulin resistance and other diabetic complications; and is involved in the antitumoral effects of cannabinoids.
But how do anandamide and 2-AG – or CBD, for that matter – get inside the cell? How are these oily compounds able to navigate the cell’s aqueous interior? How do they find their way to the nucleus, where they activate PPAR receptors, which attach to DNA “promoter” segments that initiate or prevent the transcription of specific genes?
2009: Fatty Acid Binding Proteins
Researchers at Stony Brook University in New York made huge strides in unraveling the riddle of endocannabinoid mobility in 2009 when they identified a fatty acid binding protein (FABP) that transports anandamide through the cell’s watery, internal ecosystem. These transport molecules also shuttle 2-AG and other lipid compounds to the great beyond within the cell.
After they finish signaling via a cannabinoid receptor, anandamide or 2-AG attach themselves to a FABP transporter, slip through the cell membrane’s lipid bilayer, and set sail amidst an archipelago of organelles. Fatty acid binding proteins can direct endocannabinoids to the nucleus for PPAR activation or to other locations inside the cell, where anandamide and 2-AG are ultimately deactivated and broken down into metabolites.
Stony Brook scientists subsequently discovered that the same FABPs can function as carrier molecules for CBD and THC, which also don’t mix well with water. When they hop on board this lipid transport vehicle, plant cannabinoids displace their endogenous counterparts and delay their intracellular journey. Consequently, anandamide and 2-AG hang around the surface of the cell longer than usual, which extends cannabinoid receptor signaling. In effect, CBD and THC inhibit the reuptake and postpone the deactivation of anandamide and 2-AG. This may be one of the ways that CBD, in particular, enhances endocannabinoid tone without actually binding directly to CB1 or CB2.
2012: Mitochondria
In 2012, French scientists reported the presence of CB1 receptors on the membranes of mitochondria, the energy-generating organelle within cells. This discovery shed new light on the role of the endocannabinoid system in regulating mitochondrial activity, which is critical to how cells function. Key biological pathways that involve mitochondria — including energy homeostasis, neurotransmitter release, and oxidative stress — are modulated by endocannabinoids and plant cannabinoids.
Oxidative stress is a natural byproduct of mitochondrial activity, but high levels of oxidative stress are a sign that something is off kilter in the cell. By effectively neutralizing oxidative stress and mitigating free radical damage, cannabinoids confer a broad range of therapeutic benefits — from slowing down the aging process to reducing the risk of DNA damage linked to cancer. As reported in Philosophical Transactions of the Royal Society (London): “Cannabinoids as regulators of mitochondrial activity … protect neurons on the molecular level … Neuroinflammatory processes contributing to the progression of normal brain ageing and to the pathogenesis of neurodegenerative diseases are suppressed by cannabinoids, suggesting that they may also influence the aging process on the system level.”
By binding directly to CB1 receptors on the mitochondrial membrane, THC dials back mitochondria activity and oxidative excess. CBD interacts with a different set of mitochondrial receptors, including the sodium-calcium exchanger (NCX), which opens and closes an ion channel that facilitates the flow of electrically charged calcium atoms. Regulating calcium levels inside the mitochondria is one of the mechanisms whereby endocannabinoids and CBD protect neurons and maintain cellular homeostasis. MAGL, the main enzyme that breaks down 2-AG, is conveniently stationed by the mitochondrion, while FAAH metabolizes anandamide when the bliss molecule disembarks at another organelle, the endoplasmic reticulum – the last stop on our FABP-driven tour de force through intracellular space.
2013: The Endocannabinoidome
Twenty-five years after the discovery of the CB1 receptor, the canonical endocannabinoid system as a conceptual framework was challenged by a series of new revelations. At the very least it seemed that a more expansive definition of “cannabinoid receptor” was warranted, a definition that acknowledges the three major receptor groups that bind to anandamide and 2-AG: TRP receptors and other ligand-sensitive ion channels, PPAR nuclear receptors, and several G-protein coupled receptors in addition to CB1 and CB2.
THC, CBD, and other plant cannabinoids also engage in promiscuous coupling with multiple receptor partners. What’s more, various herbs and spices, not just cannabis, contain compounds that bind to CB1 and/or CB2. Beyond herbal therapies, beneficial effects of other holistic healing modalities (fasting, exercise, osteopathy, acupuncture) are also mediated by the canonical cannabinoid receptors.
Accordingly, scientists have begun to think in terms of an “enlarged endocannabinoid system,” one that includes a raft of fatty acid-derived lipids in addition to anandamide and 2-AG. These endocannabinoid-like compounds have emerged as important signaling molecules in their own right, and some are also metabolized by FAAH, the enzyme that breaks down anandamide. In 2013, Vincenzo Di Marzo, a leading cannabinoid scientist, introduced the idea of the “endocannabinoidome,” a complex hyper-system that encompasses our innate “lipidome,” as well as our gut microbiome. Endocannabinoid signaling facilitates crosstalk between gut flora and the brain, a process increasingly recognized as fundamental to human health.
Afterword: Retrograde History
The endocannabinoid system, a physiological system of huge importance, is named after the plant that pointed the way to its discovery. “We would not have been able to get there if we had not looked at the plant,” Mechoulam acknowledged. Everything scientists know about the endocannabinoid system was made possible by the plant teacher.
It was during the 1990s, the so-called “decade of the brain,” that the basic components of the canonical endocannabinoid system were established. Since then, we have learned a lot more about the endocannabinoid system and its interactions with many other lipid signaling molecules and receptor networks beyond CB1 and CB2. We’ve learned that when the endocannabinoid system fails to function properly, plant cannabinoids can pick up the slack and provide relief. We recognize that cannabis is such a versatile therapeutic substance because it acts via cannabinoid receptors and other pathways that exist throughout the brain and body.
Cannabis emerged as a distinct botanical species almost 30 million years ago, but the endocannabinoid system has been around a lot longer than its botanical namesake. In retrospect, it appears that scientific understanding of the endocannabinoid system has unfolded backwards through time. Researchers initially focused on the plant, which evolved much later than the endocannabinoid system. And the three components of the canonical endocannabinoid system – receptors, endocannabinoids, and enzymes – were discovered in reverse order from how they actually evolved over eons.
First scientists studied the chemical constituents of cannabis. The plant led researchers to the CB1 cannabinoid receptor, which dates back to the chordate ancestor of all vertebrates more than 500 million years before cannabis flowered on the planet. Knowledge of the CB1 receptor, in turn, paved the way for scientific discovery of the two main endogenous cannabinoids, anandamide and 2-AG. These compounds were present in hydra and other primitive animals (without cannabinoid receptors) that preceded vertebrates. This indicates that endocannabinoids evolved earlier than the cannabinoid receptors.
Evolutionary biologist Maurice Elphick suggests that the third and most ancient component of the canonical endocannabinoid system – and the last to be discovered by scientists – are the enzymes that metabolize anandamide and 2-AG. The ability of cells to synthesize and break down endocannabinoids traces back a billion years to a primordial enzyme in one of the earliest life forms on earth, the common unicellular ancestor of animals and plants.
That’s what we have learned from cannabis.
Martin A. Lee is the director of Project CBD. He’s authored and edited several books, including Smoke Signals, Acid Dreams, and The Essential Guide to CBD. © Copyright, Project CBD. May not be reprinted without permission.
Recommended Readings
The Brain & Marijuana
Excerpted from “Smoke Signals: A Social History of Marijuana – Medical, Recreational, and Scientific” by Martin A. Lee.
Natural High: CBD Augments Anandamide
It has been known for some time that CBD acutely increases endogenous cannabinoid levels.
Endocannabinoids & Endocrine Disruptors
Do chemical pollutants wreak havoc by impairing the endocannabinoid system?