Chirality. A word that many readers may not be familiar with. It’s a concept relevant to the cannabis plant’s diverse pharmacopoeia of cannabinoids and terpenes, and it may be a hitherto unknown facet of how strain-specific cannabis effects work. Chirality may also hold the key to turning THC or CBD into even better therapeutics – or alternatively it might unleash some new hidden dangers.
What Is Chirality?
Chirality is an organic chemistry concept rooted in simple elements of geometry. Geometry works the same at any scale, macro or molecular, which allows for many real-world examples to help conceptualize what chirality is and what is happening at the miniscule sizes of the hidden world of chemistry.
Hold up your two hands. Are they identical? It appears that they are the same in size, the same in shape, the same in all relative proportions … But try putting a left-handed glove on your right-hand. It doesn’t fit well. Are you able to describe what it is that makes your hands different from one another?
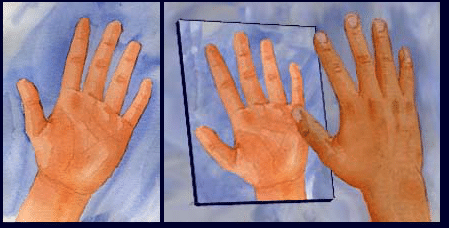
Our hands are the most universally recognized example of chirality – in fact, the word chiral originates from cheir, the Greek word for ‘hand.’ Our hands are almost identical, yet they are not superimposable. No matter how your right hand is oriented, it is physically impossible to completely align it with your left hand. Our hands are mirror images of one another.
Your right hand is inherently different from its own mirror image. This is chirality. Any object that can be differentiated from its mirror image is called chiral. A chiral object and its corresponding mirror-image are known as enantiomers. Your left hand is an enantiomer of your right hand.
Is chirality a universal property? Not at all. Visualize a basketball, a football, a teacup, a pencil – a model made from their mirror image would be completely indistinguishable from the original. These are all non-chiral objects.
A screw is chiral: Its mirror-image turns my ‘lefty-loosey/righty-tighty’ screw into a ‘righty-loosey’ one! No matter how I orient my mirror-image screw it will never be able to left-loosen like the original. The left-loosen screw and the right-loosen screw are enantiomers.
A nail is non-chiral: It doesn’t matter to my hammer whether it’s pounding the original nail or its mirror-image, because the two are virtually indistinguishable. No enantiomer exists for a nail.
Chiral Chemistry
Almost all the matter we see around us is made up of molecules. Molecules are unimaginably tiny things, yet they do truly physically exist in three-dimensional space. They have shape and substance. Like the nail and the screw, some molecules are chiral, while others similar in shape, size or function, may not be.

Chirality is something that scientists need to pay a lot of attention to while developing new medicines. Most medicines and other drugs work by having their active ingredient be a molecule which precisely ‘fits’ into a certain cellular receptor somewhere in our body. Sometimes chirality affects how a drug works, and sometimes it doesn’t. Figure #1 serves to illustrate why this may be the case.
The black glove can only be comfortably worn on the right hand, while the white glove can be worn equally well on either hand.
The black glove in Figure #1 contours in such a way where it only fits one hand, whereas the shape of the white glove is more forgiving to an ambidextrous fit. We may be able to squeeze our left hand into the black glove, but it will be less comfortable, and we will lose some functionality due to our thumb not aligning properly with the thumbhole. This same concept can be applied at a miniscule scale with how a chiral drug molecule (a hand) may or may not fit into a target cellular receptor (a glove).
Chirality’s effects are situational. It depends on the specifics of how a drug and our receptors interact:
Sometimes one enantiomer works better than another.
We can squeeze the wrong hand into the glove, but there is reduced functionality.
Sometimes only one of the enantiomers works.
The wrong hand cannot fit into the glove at all.
Sometimes which enantiomer does not matter.
Both hands fit the glove equally.
Sometimes mixing enantiomers is more efficacious.
The ADHD medication Adderall is formulated at a 3:1 ratio of right-handed to left-handed amphetamine. Lefty- and righty-amphetamine have differential effects in the body and work better when together for many patients.
Sometimes mixing enantiomers is disastrous.
Thalidomide was a morning sickness medication that tragically caused thousands of severe birth defects due to inadequate toxicity assessment of the drug’s left-handed vs. right-handed versions. One thalidomide enantiomer damages a fetus growing in the womb, while the other is totally harmless!
Chiral Cannabis
Cannabinoids, too, are chiral. Molecules of THC and CBD cannot be superimposed with their mirror images. They have ‘handedness’ but unlike our hands, cannabinoid molecules have two different manners of reflective symmetry, resulting in two separate pairs of enantiomers. You can think of a THC molecule as having properties of being ‘left-handed or right-handed’ and additionally being either ‘up-handed or down-handed’. Two pairs of enantiomers means that there are four possible forms that THC could exist in when its chirality is taken into consideration (see Figure #2).
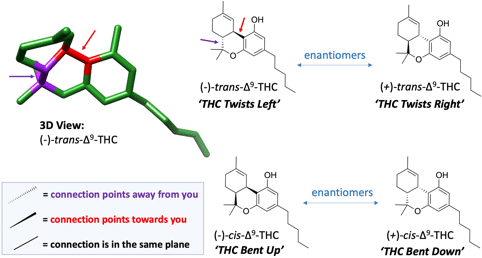
You may know the name of THC in your cannabis as ‘Δ9-tetrahydrocannabinol’, but that’s not the full truth. The full chemical name of THC is actually ‘(−)-trans–Δ9-tetrahydrocannabinol’. The (-) and the ‘trans’ prefixes specify spatial features of chirality. A molecule of THC can be either ‘(+) or (−)’ and be either ‘cis or trans.’ Though, for nearly all intents and purposes we can just say “THC” without needing any further clarification given that almost all THC on the planet exists as only a single chirality. The cannabis plant almost exclusively makes its THC as (−)-trans–Δ9-tetrahydrocannabinol. Likewise, plant-made CBD is in the form (-)-trans-cannabidiol, despite other chiralities being possible.
One-handedness is not a universal feature of all cannabinoids, however. Cannabichromene (CBC), for example, is naturally expressed as a mixture of its left- and right-handed forms at ~3:1 ratio.1,2 Multiple groups over the years have additionally reported detection of small amounts of naturally expressed (+/-)-cis-THC in hemp, but thus far cis-THC has eluded any detection in non-hemp varieties of cannabis.3,4
It should be noted though that the typical lab tests used to determine cannabis potency will not differentiate between chiralities, so looking for unusually handed cannabinoids has historically been restricted to the esoteric domains of academic laboratories and scientists working in niche corners of industry.
Chiral Pharmacology
Non-natural enantiomers of cannabinoids can be created in a laboratory, and this permits their study. Over decades of sporadic research on the topic it’s become clear that the chirality of cannabinoids is indeed an important factor influencing their effects in the body.
THC produces a psychotropic high by ‘fitting just right’ into CB1 cannabinoid receptors in our brain. Consuming CBD does not cause an overt high because the CBD molecules do not ‘fit’ very well into CB1 and therefore cannot appreciably activate these receptors. However, when famed cannabinoid scientist Dr. Raphael Mechoulam and his team in Israel created CBD’s non-natural enantiomer, (+)-CBD, it showed similar potency as regular CBD in its anti-convulsant effects but was different in that it was able to activate CB1 receptors with surprising strength!5-7
The interaction of (+)-CBD with CB1 was far stronger than regular CBD, but still ~30x weaker than THC. The group went on to create the non-natural enantiomer of the expected metabolite (aka what the human body likely processes (+)-CBD into) and found the lefty metabolite (+)-7-hydroxy-CBD bound cannabinoid receptors incredibly tightly … even stronger than THC!8 This means (+)-CBD may induce a psychotropic high, although when a moderately large dose was given to mice it did not seem to produce any THC-like effects. Nevertheless, due to differences in metabolism and route of administration there is reason to believe that (+)-CBD may have intoxicating effects in humans.
Conversely, the atypical enantiomers of THC activate the CB1 receptor comparatively much weaker than the dominant natural enantiomer (-)-trans–THC. A recent study demonstrated that (-)-cis–THC interacts about 10x weaker with CB1 receptors, and (+)-cis–THC about 100x weaker, than the naturally predominant form of THC. This seems to be in agreement with old animal studies conducted by Mechoulam and others half a century ago where (+)-cis-THC was estimated to be roughly 100x weaker in potency than natural THC by monitoring their respective behavioral effects.9,10
Chiral Terpenes
Cannabinoids aren’t the only chiral cannabis compounds – most terpenes are chiral too! Terpenes certainly aren’t unique to the cannabis plant, but cannabis is notable among the entire plant world for its abundance of terpenes, both in quantity and in how many diverse types it naturally expresses in its flower.
Plants make some terpenes exclusively in one chiral form, but more often terpenes are expressed in specific ratios of their left- to right-handed forms. This ‘enantiomeric ratio’ for a terpene tends to be similar across different plants, but not always.11-13 Chirality differences may affect a plant’s flavor, fragrance, or effects. For example, the terpene (+)-limonene smells like oranges whereas its enantiomer, (-)-limonene, smells like lemon. I say “smells like” but it would alternatively be valid to say they ARE the smell … these are literally the compounds that your nose detects and your brain deciphers as citrus peel aroma.
Terpenes provide cannabis with its distinctive aroma, and they are widely believed to be a key piece to the puzzle of entourage effects. Cannabis enthusiasts and patients may look at lab-reported terpene profiles to guide which flower they select at the dispensary. However, many cannabis terpenes are naturally found in multiple chiralities, and these lab reports don’t differentiate between terpene enantiomers.
A recent study examining terpene chirality in nine cultivars of cannabis found roughly the same ‘enantiomeric ratios’ across strains for most terpenes they looked at. But there were also some interesting differences uncovered, such as large disparities between (+)-linalool vs. (-)-linalool, and of (+)-beta-pinene vs. (-)-beta-pinene.14 Disparate effects can certainly arise from differences in terpene chirality.15 So this adds yet another layer of complexity to cannabis terpenes profiles and raises the possibility that chirality may be an additional factor driving strain-specific differences in perceived effects.
Chiral Safety
We have an overwhelming amount of anecdotal and objective evidence showing that cannabis and the major cannabinoids (THC & CBD) possess very high general safety profiles. However, >99.9% of the THC and CBD on this planet exist in only one chirality. Nearly all human experience with cannabis is with plant cannabinoids in their (-)-trans form.
We know the chirality of a molecule has the potential to greatly affect how it interacts with our bodies, but the currently available data on unnaturally handed cannabinoids is still quite sparse. Much remains unknown about their disparate effects in humans or potential for toxicity. It’s possible that some could turn out to have unique beneficial properties. Perhaps lefty-THC retains regular-THC’s safety and beneficial effects against nausea and pain without the accompanying psychoactivity that many patients wish to avoid. Who knows? While it seems unlikely that these lefty-cannabinoids would cause serious negative side effects, the possibility shouldn’t be discounted because the truth is that we really don’t know yet.
But if nearly all cannabinoids everywhere are found in the same chiral form, then why does any of this matter? Why? Because the industry trajectory is skewed towards an increased prevalence of synthetically produced cannabinoids on the horizon. With biopharmaceutical companies betting heavily on lab-created cannabinoids, the unusual chiral permutations of these compounds and their surprising effects could have significant implications for the future of cannabis medicine.
One important point needs clarification: ‘synthetic cannabinoids’ and ‘synthetically produced cannabinoids’ mean very different things.
- Synthetic cannabinoids, such as the infamous K2 and Spice, do not exist in nature. They’re compounds that were specifically developed to be a ‘shape’ which precisely fits our CB1 receptors and activate them with ferocious strength. This can mimic the feel of a THC-induced psychotropic high, but unlike THC, synthetic cannabinoids often have unpredictable side-effects and serious dangers.
- Synthetically produced cannabinoids usually mean creating the exact same cannabinoid molecule that the cannabis plant does, but our THC or CBD is being made in a test tube rather than letting the plant’s chemistry do the work. There is nothing inherently wrong about synthetically produced replicas of natural compounds so long as there are appropriate checks in place to confirm adherence to clean practices and ensure adequate purity of the product.
Synthetic production of natural compounds is already exceedingly common across many modern industries. Caffeine, for example, is naturally a plant-made compound, but the caffeine in your cola was almost certainly created synthetically in a lab rather than by a plant.
There are many positive aspects to the synthetic production of cannabinoids, particularly the prospect of being able to produce species of minor cannabinoids that are difficult or impossible to achieve in sufficient yields using classical agricultural methods. If all you need to source is a single cannabinoid (aka an isolate) rather than flower or full-spectrum cannabis oil, then synthetic production can meet that demand while eliminating most of the headaches typical of farming – no unpredictable weather, no winters, low space footprint, and no pests means no need for pesticides.
Synthetic production is how pharmaceutical THC (Marinol/dronabinol) has been made since 1986 when clinicians began prescribing it to treat chemotherapy-induced nausea. (FDA-approved pharmaceutical THC is carefully synthesized to be the same chiral form as the natural THC found in the cannabis plant.) More recently, semi-synthetic production (turning one cannabinoid into another) of certain minor cannabinoids like ∆8-THC and CBN has become relatively commonplace in the largely unregulated cannabis industry. Genetically engineering yeast to ferment sugars into cannabinoids rather than alcohols is not something out of a science fiction book, it is happening today!16 And it’s likely to play an increasingly prevalent role in the coming years with multiple biotech companies aggressively pursuing the R&D needed to create industrial ‘cannabinoid breweries’.
Chiral Challenges
Chirality can be a major challenge for companies pursuing non-agricultural methods of cannabinoid production. When synthetically producing a molecule, it’s often the case that it will spontaneously form into equal proportions of every possible chirality; in chemistry this is called a racemic mixture. This means that a significant portion of synthetically made cannabinoids may exist in a non-natural chirality.
It is indeed possible to take a racemic mixture and purify it into only one single chirality, however doing so can be an arduous and costly process. Chemists can sometimes employ tricks to coax a synthesis to only form a single enantiomer, but not always, and there are usually added costs and complexity to non-racemic synthesis of chiral compounds.
Are regulators realistically prepared to oversee the synthetic production of CBD and other cannabinoids?
While cannabis has been legalized at the State-level in many parts of the U.S., currently there is little federal supervision of the cannabis industry, including hemp-derived CBD production and its offshoots, which proliferate without FDA oversight. If a manufacturer wants to try out a potentially dangerous flavor additive in their vapes or bump up their potency with some hot new non-natural cannabinoid like THC-O-acetate or use some unregulated chemical catalyst to semi-synthetically convert their CBD into ∆8-THC – they can (and do) do it. What’s more, none of these things would be detected during standard State-mandated cannabis compliance testing, which also doesn’t differentiate between chiralities or identify non-natural cannabinoids.
It’s only a matter of time before the non-natural (+)- or cis-versions of CBD and other cannabinoids enter the market – if for no other reason than it’s cheaper and easier for someone making non-plant cannabinoids to create a racemic mixture than an enantiomerically pure one. Hopefully the non-natural cannabinoids will turn out not to have any notable side effects, and indeed some may even have real benefits. But all bets are off until we know more. Precautionary regulations and manufacturing oversight are clearly needed.
Agricultural cannabis will likely remain the predominant force within the industry, but synthetically produced cannabinoids will inevitably claim a significant share of the market in the coming years. Synthetic production can be a boon to companies and consumers alike by providing scalable access to virtually any cannabinoid, which can power new product formulations that wouldn’t be otherwise possible. But any business making non-agricultural cannabinoids should have to prove that they are 100% exactly the same chirality that we get from Mother Nature.
With the advent and adoption of new synthetic production methods, the chirality of cannabinoids is emerging as a pertinent issue for the first time in history. While exploring this fascinating new side of cannabis we should be mindful of what we have learned from our history with other chiral drugs – both the exciting potential that could make THC an even better medication for nausea than it already is, but also the potential highlighted in the tragic story of another nausea medication called thalidomide.
Dr. Matt Elmes is a cannabinoid scientist and cannabis enthusiast. His professional background spans both academia and industry. He currently works as a freelance consultant for biopharmaceutical and cannabis companies. © Copyright, Project CBD. May not be reprinted without permission.
Footnotes
- Hanus, L. O., Meyer, S. M., Munoz, E., Taglialatela-Scafati, O. & Appendino, G. Phytocannabinoids: a unified critical inventory. Nat Prod Rep 33, 1357-1392, doi:10.1039/c6np00074f (2016).
- Filer, C. N. Chirality in Cannabinoid Research. Cannabis Cannabinoid Res 6, 1-4, doi:10.1089/can.2020.0027 (2021).
- Schafroth, M. A. et al. Delta(9)-cis-Tetrahydrocannabinol: Natural Occurrence, Chirality, and Pharmacology. J Nat Prod 84, 2502-2510, doi:10.1021/acs.jnatprod.1c00513 (2021).
- Smith, R. M., & Kempfert, K. D. . Delta1-3, 4 cis tetrahydrocannabinol in Cannabis sativa. Phytochemistry (1977).
- Fride, E. et al. (+)-Cannabidiol analogues which bind cannabinoid receptors but exert peripheral activity only. Eur J Pharmacol 506, 179-188, doi:10.1016/j.ejphar.2004.10.049 (2004).
- Leite, J. R., Carlini, E. A., Lander, N. & Mechoulam, R. Anticonvulsant effects of the (-) and (+)isomers of cannabidiol and their dimethylheptyl homologs. Pharmacology 24, 141-146, doi:10.1159/000137588 (1982).
- Bisogno, T. et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 134, 845-852, doi:10.1038/sj.bjp.0704327 (2001).
- Hanus, L. O. et al. Enantiomeric cannabidiol derivatives: synthesis and binding to cannabinoid receptors. Org Biomol Chem 3, 1116-1123, doi:10.1039/b416943c (2005).
- Ben-Zvi, Z., Mechoulam, R., Edery, H. & Porath, G. 6 -hydroxy- 1 -tetrahydrocannabinol synthesis and biological activity. Science 174, 951-952, doi:10.1126/science.174.4012.951 (1971).
- Martin, B. R., Balster, R. L., Razdan, R. K., Harris, L. S. & Dewey, W. L. Behavioral comparisons of the stereoisomers of tetrahydrocannabinols. Life Sci 29, 565-574, doi:10.1016/0024-3205(81)90434-3 (1981).
- Ozek, T., Tabanca, N., Demirci, F., Wedge, D. E., & Baser, K. . Enantiomeric distribution of some linalool containing essential oils and their biological activities. Rec. Nat. Prod., 180-192 (2010).
- Tsokou, A., Georgopoulou, K., Melliou, E., Magiatis, P. & Tsitsa, E. Composition and enantiomeric analysis of the essential oil of the fruits and the leaves of Pistacia vera from Greece. Molecules 12, 1233-1239, doi:10.3390/12061233 (2007).
- Davies, N. W., Larkman, T., Marriott, P. J. & Khan, I. A. Determination of Enantiomeric Distribution of Terpenes for Quality Assessment of Australian Tea Tree Oil. J Agric Food Chem 64, 4817-4819, doi:10.1021/acs.jafc.6b01803 (2016).
- Mazzara, E. et al. A Comprehensive Phytochemical Analysis of Terpenes, Polyphenols and Cannabinoids, and Micromorphological Characterization of 9 Commercial Varieties of Cannabis sativa L. Plants (Basel) 11, doi:10.3390/plants11070891 (2022).
- de Sousa, D. P., Nobrega, F. F., Santos, C. C. & de Almeida, R. N. Anticonvulsant activity of the linalool enantiomers and racemate: investigation of chiral influence. Nat Prod Commun 5, 1847-1851 (2010).
- Luo, X. et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 567, 123-126, doi:10.1038/s41586-019-0978-9 (2019).
Recommended Readings
Project CBD Releases Primer on Cannabinoid-Drug Interactions
Physicians and patients need to understand how cannabis interacts with common pharmaceuticals.
Under the Radar: Synthetic Cannabinoids & EVALI
The CDC’s diagnostic criteria for EVALI parallel the symptoms of synthetic cannabinoid use.
Medical Cannabis Can Help America’s Opiate Crisis
A doctor discusses a neglected treatment option for opioid addiction: medical marijuana.